How batteries work: explained to my 10 years old sister
The real groundwork from chemistry to battery mechanisms, in a way everyone can understand.
12/25/202310 min read


Welcome back everyone, In this post, we'll go through the real groundwork of how batteries work. Electric vehicles (EVs) are becoming increasingly popular, with heavy support and incentives from governments worldwide. EVs can be mainly subdivided into two categories.
"Full electric", also known as BEV (Battery Electric Vehicles)
"Hybrid", also known as PHEV (Plug-in Hybrid Electric Vehicles)
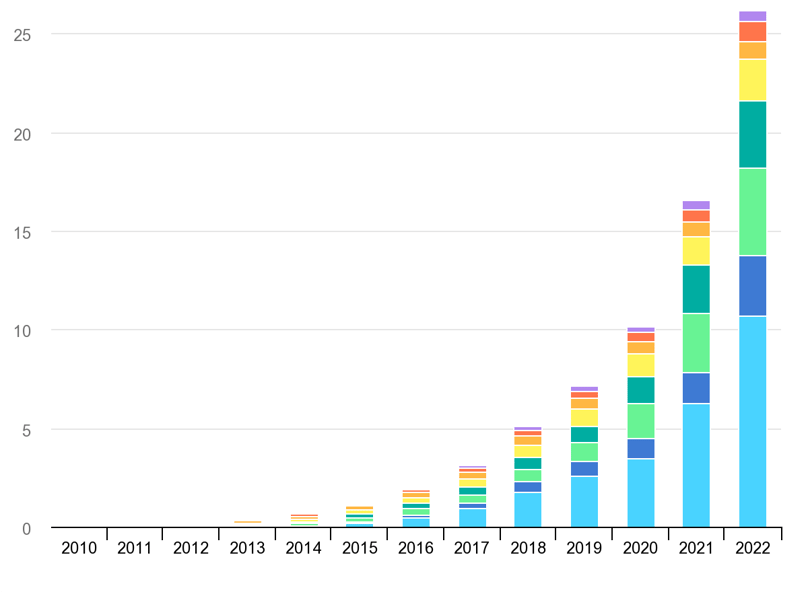
The combined sales of BEV and PHEV worldwide went from almost zero in 2010 to well beyond 25 Million units in 2022 (18.3 million BEV of which 10.7 million just in China) as you can see in the chart below.




In the image above, you can see the structure of a Lithium atom ("Li" on the periodic table, number 3). A Lithium atom is made of 3 protons (heavy and positively charged particles sitting in the nucleus of the atom), 3 neutrons (guess, heavy and neutrally charged particles sitting in the nucleus with protons), and 3 electrons (light, negatively charged particles, in constant movement around the nucleus). Yes, you probably guessed right, "Li" has number 3 on the periodic table due to this. Most precisely, the number assigned to elements in the periodic table depends on the number of protons and electrons of an element (neutrons can vary). Elements have a neutral charge, as negatives and positives cancel each other out.
Ok then, another step forward, elements are made of protons and electrons, which are electrically charged particles, and we are getting closer. We can imagine now that to have electricity, these electrically charged particles are involved, more precisely, we want them to move.
HOW do charged particles move?
For now, we understand that our planet is made of 118 chemical elements, and they're made of protons, electrons, and neutrons (charged particles, besides neutrons), and based on how many charged particles make up the element, we have different elements. To have electricity, we want some kind of movement of these particles. How do they move?
We can make a comparison with everyday objects. How do objects move? If a force is applied to them. If I push my laptop, it will move. If I push over the edge of the desk, it will fall. The laptop, by just sitting on the desk, has the potential to fall down. This "potential" becomes reality if I am silly enough to let it fall.
Just like my poor laptop, also electrically charged particles have "potential" to move, which is called "electric potential". Charged particles, just like my laptop, will move from a state of high potential to low potential.
Let's not stick too much on this vague topic of potential, just remember that charged particles move where an electric potential (or voltage) is applied.
WHERE do charged particles move?
I hope for now everything is quite clear, in order to have electricity, you need electric potential, or voltage, to make the charged particles move. But where do they move? What is the road tey take?
Well, we need to look at the structure of our materials.
Metals have a particular structure. Protons are stable and never move, whilst electrons are free to move. Electrons are weakly attracted to protons, so they just wander around, with no specific direction. Once an electric potential is applied, these electrons will all move in one specific direction (as we learned before, from high to low potential). Please have a look at the image below for simplicity.
IEA, Global electric car stock, 2010-2022, IEA, Paris https://www.iea.org/data-and-statistics/charts/global-electric-car-stock-2010-2022, IEA. Licence: CC BY 4.0
All these vehicles are fully or partially powered by batteries! Like your smartphones, alarm clocks, and many other devices for daily use. We will not delve into EV-related topics in this post, but given their recent impact on the world, I'll just explain the basics of how batteries work.
Let's start with the periodic table
Let's not worry here, I just need it for a quick reference. The periodic table of elements is no other than a big chart that helps scientists (or whoever consulting it) to know the differences among the chemical elements present on this planet.
As of 2023, there are 118 recognized elements on the periodic table. Yes, the WHOLE planet Earth is made only of 118 elements and their combinations. These elements are placed on this chart based on their properties. For example:
The elements in pink and blue are mostly metals (besides Hydrogen and Helium)
The elements in yellow are called non-metals
The green are rare earths and radioactive materials
If the elements are part of the same color code, means they have similar properties, for example, metals can conduct electricity! Ok, this is already a step forward, now we know that if we want electricity, we need to have some metals to conduct it at some point.
But let's not go too fast. The smallest unit of an element is called "atom" (which means not-divisible in Greek). Element's atoms themselves are made of even smaller particles, called subatomic particles. For the sake of simplicity let's just mention the 3 most known and relevant for this article: electrons, protons, and neutrons.
The periodic table of elements
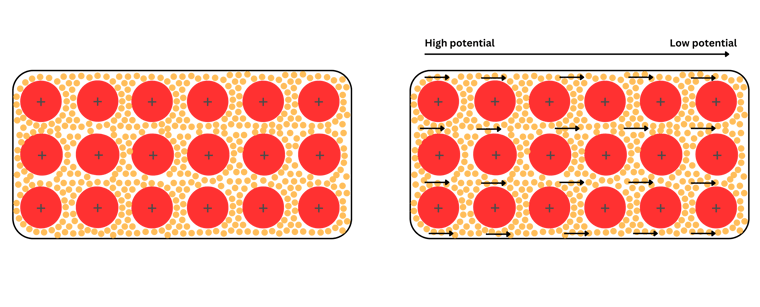
As you can see from the image, the protons of the metals are fixed, immersed In a swarm of electrons, which will start moving all in the same direction once a voltage is applied.
There you go! This is how the negatively charged particles, electrons, move to generate electricity. When you plug your phone into the socket, electrons from the power grid will flow through the metallic cable into your phone, charging your phone's battery.
Bear with me, because batteries are slightly more complicated than that. But we are getting there.
Does water conduct electricity?
We all know we can use the hairdryer with wet hands, or throw a toaster or a smartphone in a bathtub full of water. We either break the device or end up fulgurated, both preferably avoidable things. But HOW does it work?
Let's start with the water structure. We are all familiar with H2O. If we go back to the periodic table, we notice that water is made of 2 hydrogen and 1 oxygen atoms. H is the first element of the table (with number 1, so 1 proton and 1 electron), O is number 16 (16 protons and 16 electrons).
As these 2 elements are far apart (one extreme left and the other extreme right), we can assume they have very different properties. Indeed, oxygen tends to "steal" electrons and hydrogen tends to "give away" electrons.
More precisely, oxygen tends to steal 2 electrons at the same time, whilst hydrogen tends to give away 1 electron. That's why 1 atom of Oxygen binds with 2 atoms of Hydrogen at the same time, forming a molecule of water. Yes, a bunch of atoms bound togeher are called molecules.
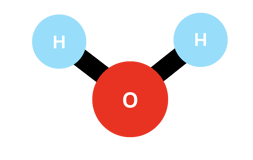
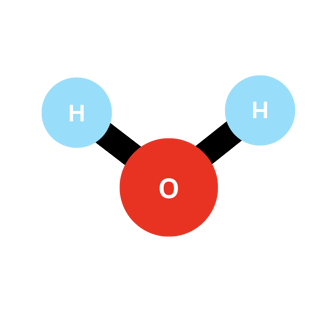
You can see above the structure of the water molecule. As you can notice, the hydrogen atoms are not aligned with the oxygen one. This is due to the remaining 16 electrons still around the oxygen atom, which occupy a lot of space and repulse the hydrogen-positive nuclei away. The 2 positive hydrogen nuclei repulse themselves as well, finally reaching this stable structure where the angle H-O-H is 104.45 degrees exactly.
Metals are solid and water is liquid (between 0 and 100 degrees C), so you can already imagine that there's no such rigid structure for liquid water as for metals.
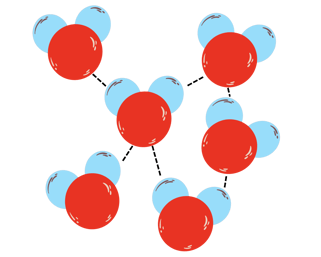
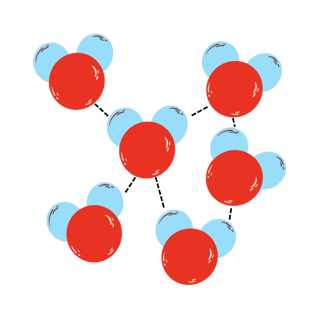
In the picture above you can see how does a water structure look like. H2O molecules loosely move around, but they're still mildly attracted one another (see dotted line, called Hydrogen bond), which keeps water liquid.
Ok, back to business, we can simplify the water molecule in the following manner. As we said, oxygen atoms are surrounded by electrons (negatively charged) and hydrogen atoms are positively charged as only the proton is exposed. We can say that even if the water molecule is neutral, the charge is not evenly distributed. The molecule it's slightly polarized, and we can simplify it as below.
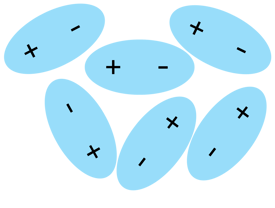
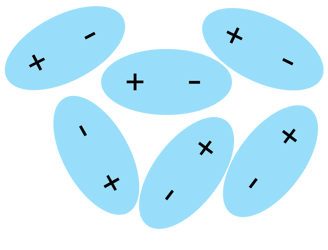
You can see above some slightly messy placed molecules slightly attracted to one another but still free to flow. As you probably already guessed, there is no such thing as a "swarm of electrons" free to flow. PURE WATER alone does not conduct electricity.
Then why do we get fulgurated if we use the hairdryer under the shower (please never do it)?
Well, water from our tap is not pure water. Our government adds some products to disinfect water to make sure water is actually drinkable and doesn't get contaminated on the way home. So, adding "something" to the water makes it conduct electricity.
Guess what, I'll explain the mechanism below. I promise all this is important to understand how batteries work.
I'll make an example that most of us know (especially Italians). When we add salt to the water to make pasta, we see salt disappearing in the water. How come?
Usual cooking salt has this chemical formula: NaCl, which means it is composed of one atom of sodium and one of chlorine. As you can see back in the periodic table, Na (sodium) and Cl (chlorine) are even farther apart than H and O. Cl tends to "steal" one electron, Na tends to "give away" 1 electron and they combine together. NaCl is a quite polarized molecule as well, even more than H2O.
When NaCl (again, kitchen salt) is in water, something like the graphics below happens:
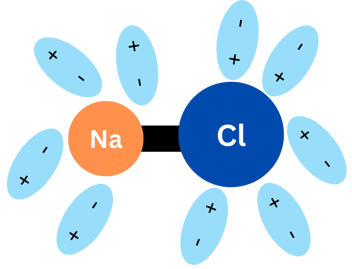
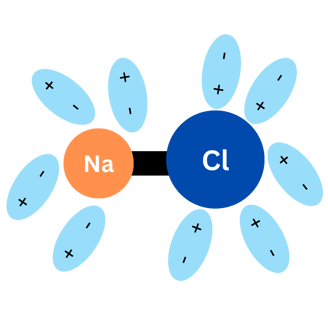
Water molecules surround NaCl molecules. The negative pole of water molecules surround the sodium and the positive pole of water will surround chlorine. This process is called "solvation" and the water molecules end up separating Na and Cl! They will be separated in Na+ and Cl-.
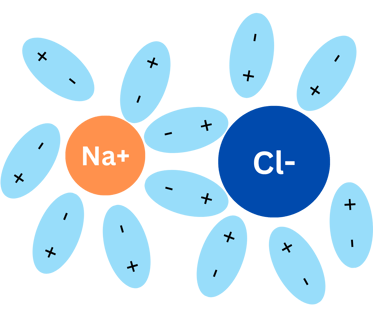
So what happened? Water molecules separate the salt molecule into two charged particles, called ions: Sodium (positively charged, +) and Chlorine (negatively charged, -).
Now, we have a liquid with charged particles floating in it. We can then correctly assume that if we apply an electric potential or voltage, these charges will move! Electricity here as well!
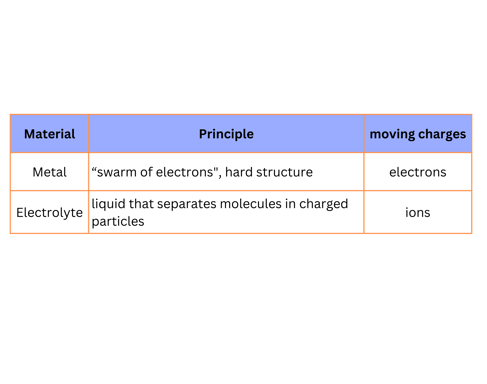
This explains why non-pure water conducts electricity. Differently from metals though, the charges moving are not electrons, but ions. Let's call this "conductive water", an electrolyte. Sounds familiar? Let's summarize with a table:
Finally, how a battery is structured
Thanks for bearing with me until this point. Now everything will be very clear, I hope.
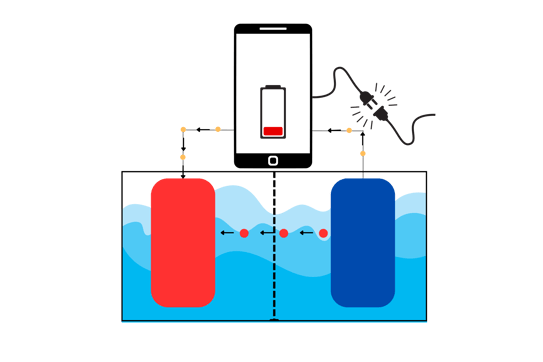
A battery is a device that stores and releases electrical energy. It is generally built as in the sketch below.
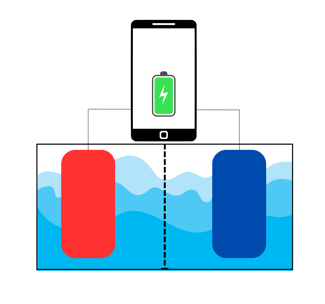
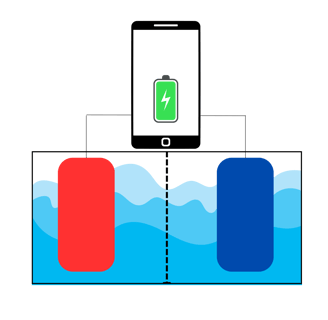
Let's pretend that in the image above, that phone is connected to a very weird battery, which is that tank under it. In the tank (the battery), the red and the blue squares are called electrodes. The red one is called anode and the blue one is cathode. The liquid they're immersed in is the electrolyte. The phone is supposedly powered by this flamboyant battery.
The anode and cathode are made of different materials. The anode is one of those materials on the left of the periodic table (typically Lithium), that tends to "give away" an electron. The cathode, at the contrary, tends to "steal" its electron.
Let's remind again: electrons travel through metals and ions travel through the electrolyte.
Now, we should have all the information and basic nomenclature to understand the following process:
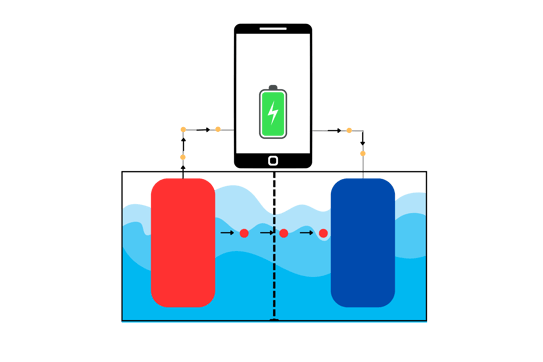
The material at the anode spontaneously (very important!) gives away its electron, which travels through the metal wire and at the same time, releases the positively charged ion in the electrolyte. The electron travels through the wire to the circuits inside the phone, making it work. The electron then flows out of the phone through the wire and ends up at the cathode.
The positively charged ion, released in the electrolyte, is attracted by the cathode. Why? Because as the cathode receives the electron from the phone (and the anode), it becomes negatively charged. The positive ion then travels towards the cathode and stops there.
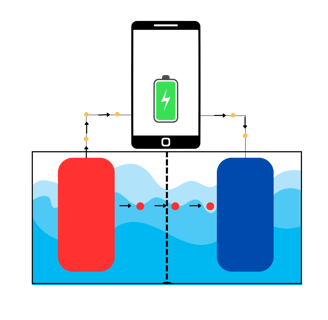
Unbelievable, but I could go on for way longer to discuss structure, details, and the difference between chargeable batteries and non-rechargeable ones. Or even focus on Li-ion battery type, different kinds of electrodes, etc... But for this post, we will stop here.
You hopefully bore with me until this point, and you learned the basic principles of how batteries work! As you might have noticed, it is a complex process, but I hope I made it clear enough for you to have a grasp of it.
I taught my sister how batteries work in this way when she was 10 years old, and we built a small battery using a lemon as an electrolyte! Maybe I will make one post to describe this easy and fun experiment to make at home with children.
Stay tuned for the next posts!
As you can see in the image above, the red balls that are going to the cathode are the positively charged ions, directed from the anode to the cathode. The yellow balls going through the wire, light up the phone and then flow to the cathode.
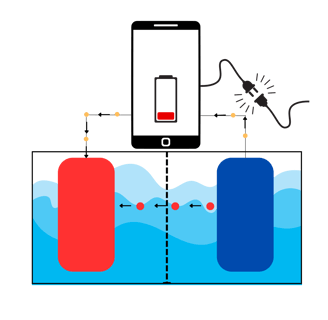
Will the anode keep providing positive ions and electrons? Until it finishes most of the ions, yes.
How to put those positive ions back into the anode to make the battery work again? Easy, we force them. We force them by applying a voltage (potential difference), in the opposite direction. In that case, the cathode will simply give back the ions to the anode. We do so by plugging the phone (the battery) into the socket!
